A Tailorable Nanotube, Formed by a Ring-shaped Protein
Contact: Dan Krotz, (510) 486-4019
There’s a new building block in the nanoscience tool box. Scientists at Berkeley Lab’s Molecular Foundry used a recently discovered, donut-shaped protein to build customizable nanotubes that can be lengthened, shortened, or sealed at both ends to form capsules.

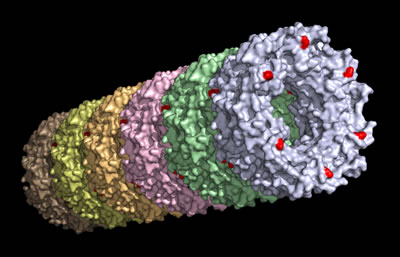
A research team that includes the Molecular Foundry’s Ron Zuckermann developed self-assembling nanotubes that can be customized in a number of ways. The scientists utilized a protein discovered in 2006 (shown on the computer monitor) that is relatively easy to express and manipulate. Six Hcp1 proteins combine to form a donut-shaped ring, which can be stacked on top of each other to form a tube. The binding sites used to fashion the rings into tubes are red.
Although still in the early investigational stage, these tailorable nanotubes could someday be used in a number of applications in which the ability to manipulate a nanotube’s length and chemical properties is critical. Perhaps they’ll be used as scaffolds to guide the construction of nanoscale wires and other devices. Or perhaps they’ll be used as drug-delivery vehicles that target disease at the molecular scale.
The nanotube’s versatility stems from the unique characteristics of its protein building block. The protein, Hcp1, naturally forms donut-shaped structures, which in turn can be stacked on top of each other like a toddler’s set of toy rings to form a tube. In addition, the nanotube’s interior and exterior can be chemically modified to bind with specific molecules, giving it a powerful functionality.
“We can work with this protein almost like an engineer,” said Ron Zuckermann, who is the Facility Director of the Biological Nanostructures Facility in the Molecular Foundry. “The protein also possesses a hierarchical structure: six of them first come together to form a ring, then these rings come together to form a tube. This is unusual and potentially very useful for building nanoscale, biologically inspired structures.”
Zuckermann developed the nanotube with Edward Ballister, also with the Molecular Foundry; Joseph Mougous of the University of Washington; and Yifan Cheng and Angela Lai of the University of California at San Francisco.
Their work has its origins in a discovery Mougous made in 2006. While studying a bacterium called Pseudomonas aeruginosa, which is implicated in pneumonia and other diseases, he came across a never-before-seen protein called Hcp1. The protein’s function in the bacterium isn’t known, but its crystal structure caught Mougous’ attention: six of the proteins link together to form a donut-shaped ring. Further examination revealed that these rings stack top to bottom to form a honeycomb lattice, which is very suggestive of tube formation.
Although puzzling from a microbiology perspective, Mougous realized that the protein’s inclination to assemble itself into a complex structure could be useful in the burgeoning field of nanoscience, which is on the lookout for building blocks that are easy to manipulate. With this in mind, he came to Berkeley Lab’s Molecular Foundry, a U.S. Department of Energy national user facility aimed at the development of nanotechnology.
Scientists who assemble nanostructures love to find building blocks like the Hcp1. Add a few cysteines here and there, create the optimal conditions, and voila: the donut-shaped proteins form tailorable nanotubes that can be lengthened, shortened, or adorned with molecules that give the tubes specific functions.
At the Molecular Foundry, the team steered the protein’s tendency to self-assemble toward the development of nanotubes. First, using a technique called mutagenesis, they substituted two amino acids that reside on the top and bottom of the donut-shaped structure with cysteines, which are amino acids that can form disulfide bonds. Next, they optimized the conditions that foster disulfide bond formation, coaxing the rings to bind together in solution — one on top of the other — courtesy of the specially engineered cysteines.
“They line up just as we’d hope they would and form a tube. There are lots of ways for this to go wrong, but with this protein it works,” said Zuckermann.
The result is a self-assembling nanotube with an interior pore that measures four nanometers in diameter (one nanometer is one-billionth of a meter). With this success, the team next tackled customization. They developed ways to control a nanotube’s length using a specially prepared Hcp1 protein that prevents the tube from adding more rings. This allows the scientists to change the nanotube’s length and therefore its interior volume. They developed nanotubes that are different at each end. And they developed large molecules that plug both ends of the nanotube, creating a capsule-like chamber.
“With these developments, we can start thinking about encapsulating something inside the nanotube, and placing targeting molecules on the outside that determine where it goes, which would be required in a drug-delivery vehicle,” said Zuckermann.
The promise of using protein nanotubes to attack disease, or serve as nanoscale construction scaffolds, is due to the relative ease with which the building block, Hcp1, can be expressed and manipulated. Hcp1 enables the construction of nanotubes that can be structurally customized and chemically modified. They can be adorned with molecules, and they are potentially compatible with biological systems — two traits that are difficult to achieve in carbon and inorganic nanotubes.
“The protein is great for bionanoengineering. All of the information needed to construct a complex structure, like tailorable nanotubes, is embedded in the protein,” said Zuckermann.
The scientists’ research is detailed in a study entitled “In vitro Self-assembly of Tailorable Nanotubes from a Simple Protein Building Block,” which was reported in the March 11, 2008 issue of the journal Proceedings of the National Academy of Sciences. The research was supported in part by the Department of Energy.
Additional Information
- More on the Molecular Foundry
- More of the Molecular Foundry’s Biological Nanostructures Facility

 Subscribe
Subscribe
 E-mail a friend
E-mail a friend
