Research Highlights
December 2007
Molecular Crash Test
Scientists slam small proteins onto a surface to see how fast the molecules fall apart
Results: Like crash test dummies in a new car ad, peptides were flung onto a surface to see how fast they would come apart. By accurately measuring the energetics and dynamics of the fragmentation of proteins, researchers at Pacific Northwest National Laboratory and the University of Hong Kong showed that a disputed theory—which provides the cornerstone of today's mass spectrometry research—accurately predicts the rate at which the protein falls apart or dissociates.
The proteins of interest are peptide radical cations; that is, short proteins containing a positive charge and missing an electron.
Why does it matter? For decades, scientists have argued whether or not the statistical theory, built in the 1920s and known as RRKM, adequately describes how fast large molecules fragment. This theory states that added energy is randomized in the molecule before it breaks apart. Although this may seem like an esoteric argument, resolving it is necessary to expand the scientific foundations of modern mass spectrometry.
"This is the first real approach to show that fragmentation of radical cations of peptides can be adequately described by statistical theory," said Pacific Northwest National Laboratory’s Julia Laskin, the study’s principal investigator.
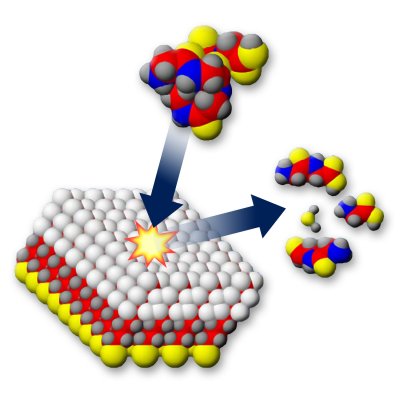
By accurately measuring the energetics and dynamics of the fragmentation of proteins, scientists at Pacific Northwest National Laboratory and the University of Hong Kong showed that a disputed theory predicts the rate at which a protein dissociates. Enlarge image
Method: To determine if the RRKM theory accurately predicted the dissociation pathways for a complex molecule, the researchers conducted careful experiments and compared the results to those predicted by the theoretical calculations. These experiments were done at the U.S. Department of Energy’s Environmental Molecular Sciences Laboratory, a national scientific user facility at PNNL.
To start, the research team needed to build the peptide radical cations. The researchers started with a short protein backbone, adding a copper complex of an organic compound. The entire complex was transferred into the gas phase. Next, an electron was moved from the peptide to the copper ion, creating a positive charge and missing electron (radical) on the peptide. An additional fragmentation step of the newly formed ion resulted in the formation of a new peptide radical cation where the radical’s location was well defined.
This carefully prepared ion is then slammed onto a surface, which adds vibrational energy to the molecule very quickly and causes fragmentation.
“The process is similar to building a stunning vase and then dropping it on the floor to see how durable it is,” said Laskin.
Using sophisticated equipment, the researchers followed fragmentation of the ions after the charged particles collided with the surface. Then, the research team conducted calculations using the RRKM theory to compare experimental and theoretical rate constants.
The researchers proved the RRKM modeling completely accounts for the kinetics of the different dissociation pathways observed in the experiments and the dissociation of the peptide radical cation is well described by the RRKM model. Preliminary results suggest that the statistical behavior is quite common for dissociation of peptide radical cations.
Next steps: The team plans to compare their results from studies of the energetics and dynamics of positive and negative odd-electron peptide ion fragmentation to the energetics and mechanisms of fragmentation of the corresponding positive and negative even-electron ions, which is much better understood.
Acknowledgments: This study was supported by the Chemical Sciences Division, Office of Basic Energy Sciences, U.S. Department of Energy and a grant by the University of Hong Kong and the Hong Kong Research Grant Council, Special Administrative Region, China.
Citation: Laskin, J., J. H. Futrell, and I. K. Chu. 2007. "Is Dissociation of Peptide Radical Cations an Ergodic Process?" Journal of the American Chemical Society. 129: 9598-9599.
